Therefore the ion of selenium has the charge of 2-. Selenium is in group 6A and is a cousin to sulfurItexhibits the -2 charge as selenide b.

Selenium Chemical Element Britannica
Chemistry Matter Net Charge.

. Se-2 selenide ion. What ion will be formed by the selenium atom shown below when it has a stable set of valence electrons. A 2 B 1 C -1 D -2 E -3 O 2 0-1 -3 O 1 O-2.
1 Answer MeneerNask Dec 18 2016. Charges of Bromine ions. Write two different formulas for an ionic compound of copper and nitrate.
Charges of Selenium ions. Se2-Which of the following is the best description of how electrons are transferred in an ionic bond. Its known as selenide ion.
Its easier for it to accept 2 valence electrons than donate its 6 valence electrons which requires higher energy. What ion will be formed by the selenium atom shown below when it has a stable set of valence electrons. What is the charge on the ion formed by selenium.
See the answer See the answer See the answer done loading. This problem has been solved. Charge of Rubidium ion.
93 rows When atoms gain electrons the negatively charged ion is formed and when the atoms lose electrons the positively charged ion is formed. CuNO 3 and Cu NO 3 2. 34 Naming Ionic Compounds Multiple-choice questions 15.
Similar to sulfide in aqueous solution the selenide ion Se2 is prevalent only in very basic conditions. Charge of Krypton ion. What is the charge on the stable ion formed by selenium.
To do this it will have to gain 2 electrons hence the 2- Note. When an atom gain electrons it becomes negatively charged therefore the charge is 2- Ions Formed By Selenium. 2- 4 6 35.
Who are the experts. Charge of Strontium ion. Lost 3 electrons b.
Copper has two common ion charges Cu and Cu2. Selenium behaves much like sulphur. Ionic Charges of all Elements List.
1- 1 5 36. HERE THE ANSWERS 2- selenium has 6 valance electrons therefore in order to be electronically stable it wants to gain two electrons in order to have a full shell of 8 valance electrons. Write the symbol or formulas with charge for each of the following ions.
Select the correct name of S2. What ion will be formed by the selenium atom shown below when it has a stable set of valence electrons. In neutral conditions hydrogen selenide ion HSe is most common.
The anion is favored. A selenide is a chemical compound containing a selenium anion with oxidation number of 2 Se2 much as sulfur does in a sulfide. Name the ions formed by the following elements a.
Show transcribed image text Expert Answer. Common ions are Se2- Se2 Se4 Se6. Se can form an anion of -2 or a cation of 6.
Ions Formed By Selenium. Its outer shell with 8 electrons the octet rule. It can aslo like sulfur form oxyacids such as selenic acid ans selenous acid H2SeO4 and H2SeO3 respectively in which its oxidation states are 6 and 4 respectivelyIn his case the only.
How many electrons were lost or gained to form these ions. A metal atom loses electrons and a nonmetal atom gains electrons. What ion will be formed by the selenium atom.

Selenium Ion An Overview Sciencedirect Topics
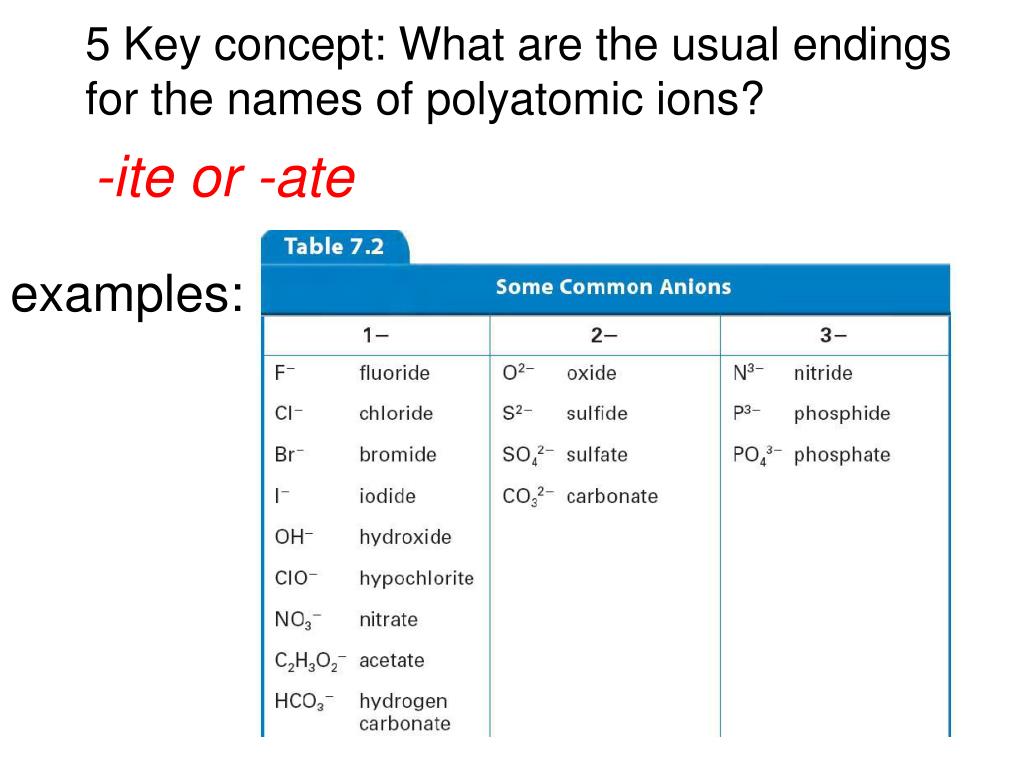
Ppt 1 Name The Ions Formed By These Elements And Classify Them As Anions Or Cations Powerpoint Presentation Id 644301

Ppt 1 Name The Ions Formed By These Elements And Classify Them As Anions Or Cations Powerpoint Presentation Id 644301
0 Comments